Sustainable Thermal Packaging for UK & Ireland Clinical Trials
TOPA THERMAL Introduces Connex ECO: Cold Chain Container with Sustainable Cellulose Wall Technology
As a leading pharmaceutical cold chain innovator, Topa Thermal is proud to show its latest advancement in sustainable thermal packaging solutions at the 2024 annual Outsourcing in Clinical Trials UK & Ireland event. The new product, Connex ECO, addresses the need for robust, eco-friendly thermal packaging when transporting temperature-sensitive pharmaceuticals during clinical trials.
Richard Harrop, Topa Thermal Product Director:
“Connex ECO isn’t your typical cardboard box or flimsy paper bag thermal packaging. It’s the smart choice for pharmaceutical companies, clinical trial organisations, CRO/CDMOs, and other specialist pharmaceutical distributors seeking a reliable, eco-friendly thermal packaging solution.”
With its intuitive bio-based rigid cellular walls, Connex ECO meets the rigorous demands of transporting temperature-sensitive specialist pharmaceuticals, clinical trial products, and cell and gene therapy treatments.
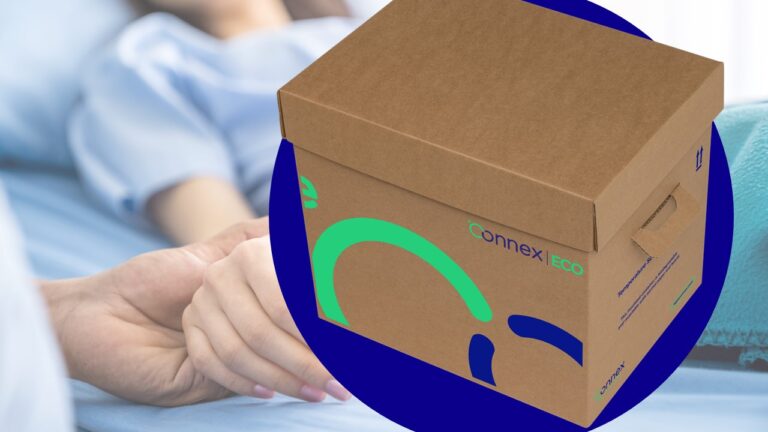
Key Features of Connex ECO:
- Plastic-Free and Bio-Based:
Connex ECO is a zero-waste thermal packaging solution specifically designed for cold chain products. Leveraging cutting-edge cellulose wall technology, it is 100% bio-based and completely free from plastic materials. By choosing Connex ECO, pharmaceutical companies and clinical trial organisations can contribute to a greener future.
- Rigid-Wall Cellulose Technology:
Unlike typical cardboard boxes or flimsy paper bags, Connex ECO features bio-based rigid cellular walls. These walls are engineered to meet the rigorous demands of transporting temperature-sensitive pharmaceuticals, clinical trial products, and cell and gene therapy treatments. With Connex ECO, you can trust that your valuable cargo remains protected throughout its journey.
- Easy Recyclability:
Connex ECO not only ensures the integrity of temperature-sensitive clinical trial products during transport but also supports environmental responsibility. After use, it can be conveniently recycled curbside along with normal paper and board materials, by clinicians, doctors, patients, and other clinical trial end-users.
- Pre-Qualified for Clinical Trial Cold Chain:
From drug manufacture to final delivery at the clinical trial site, Connex ECO maintains product efficacy while offering a plastic-free, recyclable thermal packaging solution. Its pre-qualification ensures compliance with the stringent requirements of clinical trial cold chain logistics.
Part of the Expanding °Connex Range:
Connex ECO is part of Topa Thermal’s expanding range of pre-qualified and customised temperature-controlled packaging solutions. These solutions are meticulously designed to meet the unique demands of the clinical trial industry, minimising waste and maximising efficiency.
Visit Topa Thermal at Outsourcing Clinical Trials UK & Ireland:
If you’re interested in learning more about Connex ECO and other innovative thermal packaging solutions, we invite you to visit Topa Thermal at booth 6 during the Outsourcing Clinical Trials UK & Ireland event. The event will take place on 11-12 June at Hilton Park Lane, London. Discover how Connex ECO can optimise your clinical trial logistics while contributing to a sustainable future.
About Topa Thermal
Topa Thermal is a trusted partner to pharmaceutical and distribution companies worldwide. Our advanced packaging solutions ensure the safe transport of temperature-sensitive products, maintaining efficacy and patient safety. With a commitment to excellence, we continue to redefine the boundaries of thermal packaging.
About OCT UK & Ireland 2024
Outsourcing in Clinical Trials UK & Ireland 2024 – organised by Arena International Events Group – brings together professionals from pharmaceutical companies and biopharmaceutical firms. It provides an opportunity to explore operational challenges and innovations in clinical development within the UK & Ireland region.
Outsourcing in Clinical Trials UK & Ireland 2024 will take place from June 11 to 12, in London, England.
